🔍 Introduction
Sterilization is one of the most essential practices in microbiology laboratories. It refers to the process of completely eliminating or destroying all forms of microorganisms — including bacteria, spores, fungi, and viruses — from laboratory instruments, media, and work surfaces. Without sterilization, even the most carefully planned experiments can become useless due to contamination.
Imagine preparing a bacterial culture but unknowingly introducing unwanted microbes because your instruments weren’t sterile. The result? Wrong data, wasted time, and potential hazards. That is why mastering sterilization methods is the first step toward accurate and safe laboratory research.
In this article, we will explore:
- The meaning and importance of sterilization in microbiology
- Different methods of sterilization (with examples)
- Advantages and limitations of each technique
- Why sterilization is crucial for lab safety and research accuracy
🧴 Importance of Sterilization in Microbiology
Sterilization isn’t just a formality; it plays a critical role in laboratory work:
- Prevents Contamination – Unwanted microbes in culture media can spoil experiments.
- Ensures Accuracy – Reliable results require sterile conditions.
- Protects Lab Workers – Destroys pathogens that may otherwise cause infections.
- Maintains Lab Standards – International biosafety protocols demand proper sterilization.
- Supports Public Health – Sterile techniques prevent the accidental spread of harmful organisms.
Simply put: No sterilization = No science.
🔥 Methods of Sterilization in Microbiology
Different types of microorganisms require different sterilization approaches. Below are the major sterilization techniques used in microbiology laboratories:
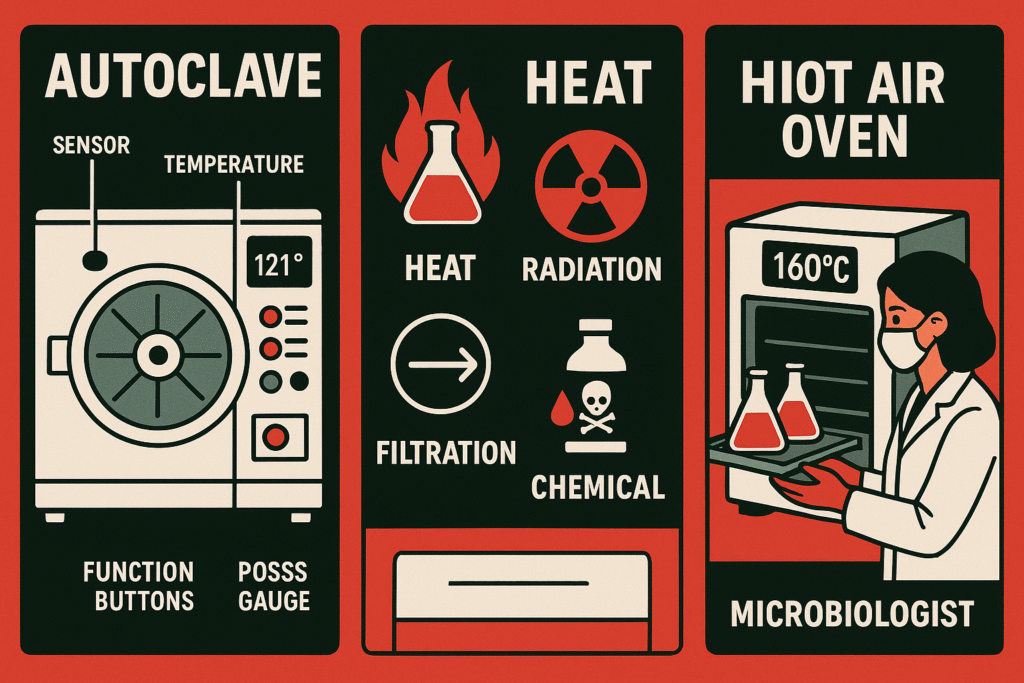
1. Autoclaving (Moist Heat Sterilization)
Autoclaving is the most common and reliable method of sterilization in microbiology labs.
- Principle: Uses steam under pressure to kill all forms of microbes, including spores.
- Standard Condition: 121°C temperature, 15 pounds per square inch (psi) pressure, for 15–20 minutes.
- Uses: Sterilization of culture media, glassware, surgical instruments, and biohazardous waste.
✅ Advantages: Fast, effective, and can handle large volumes.
❌ Limitations: Cannot be used for heat-sensitive materials (e.g., antibiotics, enzymes).
2. Dry Heat Sterilization (Hot Air Oven)
When materials cannot withstand moisture, dry heat is used.
- Standard Condition: 160–170°C for 2 hours.
- Uses: Glass Petri dishes, flasks, pipettes, oils, and powders.
✅ Advantages: Safe for materials that rust in moist heat.
❌ Limitations: Takes longer and requires higher temperatures compared to autoclaving.
3. Filtration Sterilization
Some biological fluids like antibiotics, vaccines, and serum are heat-sensitive and cannot be exposed to high temperatures.
- Method: Uses membrane filters with pore sizes of 0.22 µm to remove microorganisms.
- Uses: Heat-sensitive solutions and intravenous fluids.
✅ Advantages: Preserves sensitive compounds.
❌ Limitations: Does not remove viruses or prions effectively.
4. Radiation Sterilization
Radiation is used for both surface and deep sterilization.
- Ultraviolet (UV) Radiation:
- Used for sterilizing work surfaces, laminar flow hoods, and lab air.
- Effective but only on surfaces directly exposed to light.
- Gamma Radiation:
- Penetrates deep and sterilizes disposable plastic ware, medical devices, and pharmaceuticals.
✅ Advantages: Works for materials unsuitable for heat or chemicals.
❌ Limitations: Expensive equipment, potential safety hazards.
5. Chemical Sterilization
When heat or radiation isn’t suitable, chemical disinfectants are used.
- Examples:
- 70% Ethanol (surface sterilization)
- Sodium hypochlorite (bleach) for decontamination
- Formaldehyde gas for fumigation of lab rooms
- Uses: Surfaces, instruments, and certain sensitive materials.
✅ Advantages: Easy to apply, effective on surfaces.
❌ Limitations: Toxic fumes, requires proper ventilation and safety measures.
⚖️ Choosing the Right Sterilization Method
The selection of a sterilization technique depends on:
- Type of material (heat-resistant vs. heat-sensitive)
- Nature of microorganisms (bacteria vs. spores vs. viruses)
- Volume and purpose of material
- Cost and availability of equipment
Example:
- Glassware → Dry heat sterilization
- Culture media → Autoclaving
- Antibiotics → Filtration
- Disposable syringes → Gamma radiation
🛡️ Best Practices for Effective Sterilization
- Always check autoclave tape/indicators to confirm sterilization.
- Do not overload autoclaves or ovens; steam/heat must circulate properly.
- Replace filters regularly for effective filtration.
- Store sterilized items in dust-free and contamination-free areas.
- Train lab staff to handle sterilization equipment safely.
✅ Conclusion
Every successful microbiology experiment starts with sterile tools and sterile conditions. Whether you are preparing culture media, sterilizing glassware, or disinfecting lab benches, choosing the right sterilization method is essential.
By mastering techniques like autoclaving, dry heat sterilization, filtration, radiation, and chemical sterilization, researchers ensure both accuracy and safety. Remember, sterilization is not just about killing microbes — it is about creating an environment where science can thrive without interference.